Abstract

Introduction: Ruxolitinib (Rux) has been recently approved as second-line therapy in patients (pts) with Polycythemia Vera (PV) resistant/intolerant to hydroxyurea (HU). Median age of PV pts enrolled in the pivotal Response trials was around 60 yrs; at present, no data is reported on the use of Rux in elderly pts.
Aims: In a real-world cohort of PV pts treated with Rux, we investigated whether the efficacy and safety of Rux were comparable in pts who initiated therapy when aged ≥75 years compared with younger pts.
Methods: After IRB approval, clinical/laboratory data of 934 WHO2016-defined PV pts followed in 29 Hematology Centers were retrospectively collected. Of them, 168 (17.9%) were considered resistant/intolerant to HU at any time during follow-up by responsible physician and shifted to Rux as second-line therapy.
Results: Among the 168 pts treated with Rux, 42 (25%, median age 78.2 years) were aged ≥75 yrs at Rux start, 74 (44%, median age 67.7) were aged 60-74 and 52 (31%, median age 53) were <60 at Rux start.
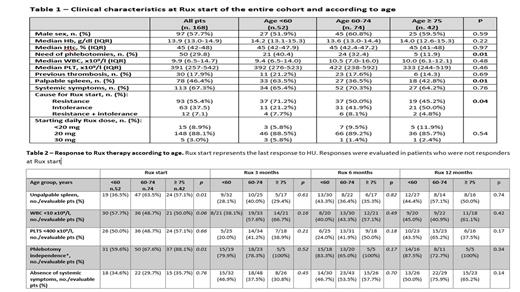
No significant differences were observed between the 3 groups, apart from a lower need for phlebotomies in pts aged ≥75 yrs and lower presence of palpable spleen in older pts (age ≥60), that more frequently switched to Rux due to HU intolerance (Table 1).
Median duration of HU treatment was 41.0 months (IQR 14.6 - 85.8), with a trend for a longer median treatment duration in pts aged ≥75 [61.0 months (IQR 21.5 - 89.6) vs 35.9 months (IQR 13.4 - 79.6), p=0.04]. Rux starting dose was similar across age groups; however, more elderly pts underwent Rux dose reductions during follow-up (45.2% in pts aged ≥75 vs 28.6% in younger pts, p=0.04).
Responses during Rux therapy are reported in Table 2, with no significant differences between the 3 groups at any time. In the overall cohort, response on PV-related symptoms at 6 and 12 months was significantly higher in pts who switched to Rux because of HU intolerance; however, this difference was not observed in pts aged ≥75 yrs.
As to the most common hematologic Rux-related toxicities, grade 3-4 anemia and thrombocytopenia were observed in only 2 (1.2%) and 5 (3%) pts, with no difference across age groups (p=0.45 and p=0.18). However, any grade anemia and thrombocytopenia during Rux were more frequently observed in pts aged ≥75 (68.3% vs 51.7% of anemia, p=0.06 and 12.2% vs 3.5% of thrombocytopenia in younger pts, p=0.04). Nineteen and 4 pts experienced infectious and thrombotic complications during Rux with incidence rates of 0.59 and 0.12 per 100 patient-months, respectively, comparably in younger and older (≥75) pts (p=0.75 and p=0.29, respectively). Notably, 6 infections were herpes simplex/zoster virus, comparably distributed between the 3 groups (p=0.60).
Permanent Rux discontinuation was needed in 14 pts (8.3%) after a median Rux exposure of 7.8 months (IQR 4.6 - 17.6) (incidence: 0.41 per 100 pts/months). Discontinuation was comparable between age groups, with Rux stop in 4 pts aged ≥75 yrs and 10 younger pts (2.4% vs 5.2% at 8 months, log-rank p=0.75). At last follow-up, 2 pts had died (1 from 2 nd neoplasia after 19.1 months from Rux start and 1 from acute leukemia after 3.3 years), both pts aged 60-74 yrs.
Conclusions. In this real-world analysis, use of Rux in HU resistant/intolerant elderly PV pts was effective and safe despite the more frequent need for dose reductions. Older age should not discourage Rux therapy, but stricter hematological monitoring may be suggested.
Latagliata: BMS Cellgene: Honoraria; Pfizer: Honoraria; Novartis: Honoraria. Breccia: Pfizer: Honoraria; Incyte: Honoraria; Bristol Myers Squibb/Celgene: Honoraria; Abbvie: Honoraria; Novartis: Honoraria. Bonifacio: Amgen: Honoraria; Bristol Myers Squibb: Honoraria; Novartis: Honoraria; Pfizer: Honoraria. Cavo: Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Consultancy, Honoraria; GlaxoSmithKline: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Accommodations, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Speakers Bureau; Bristol-Myers Squib: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Palandri: AOP: Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Membership on an entity's Board of Directors or advisory committees; CTI: Consultancy; Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal